Moving from Paper-Based Data to EDC
Overview
As the biopharmaceutical industry continues its transition to electronic data capture (EDC), the days of paper-based clinical trials are numbered. Driving this trend are factors such as lengthy study start-up time, high incidence of queries and drawn-out database lock, all linked to inefficient paper-based processes. Fountayn addresses these challenges by improving data quality and making them visible faster, enabling real- time safety reviews, and allowing sponsors and contract research organizations (CROs) to better assess the safety profile of a drug as it is being developed.
These well-established benefits of EDC have resulted in an estimated 60% of new clinical trial starts using the technology, with even higher rates of adoption in pivotal studies. But for companies still on the sidelines, still conducting clinical trials on paper, a short overview of Fountayn uEDCTM features and a case study on the paper-to-EDC migration process are presented. The case study is from a major biotechnology company, which experienced the following positive outcomes:
Immediate elimination of queries of omission
Proactive handling of serious adverse events (SAEs)
Significantly shorter time to database lock
Why EDC? Why Now?
Clinical trials are increasingly global, complex and lengthy, and as a result, companies require tools to facilitate the electronic collection of data. Continuing with paper case report forms (CRFs) leads to common errors, namely skipped fields, illegible writing and incorrect information, such as unreliable values for blood pressure or body weight. In addition, paper CRFs need to be reviewed by a study monitor, have queries resolved, and have their information entered manually into the study database. This method is notoriously slow and creates a situation in which weeks or months could elapse before data are available to sponsor or CROs, and safety signals detected. Moreover, when studies are paper-based, it is challenging to manage the growing volume of long- term safety data and comply with evolving regulatory requirements for timely reporting of SAEs.
This scenario has been documented extensively since the late 1990s to make the case for EDC, but what has changed is the quality and broad-based capabilities of today’s EDC solutions. They have become simpler, more complete and an integral part of eClinical platforms designed to streamline multiple aspects of global clinical trial programs. Some of the functions offered by The Fountayn Platform include management of patient data collected on electronic forms, visit scheduling and quick query turnaround.
Clients can also create company-specific and study specific checklists, as well as customizable workflows. All of this function and more is available from a single login with secure, permission-based access. Study data collected in the Fountayn Platform solution are stored in a central database that is accessible in real time and can be shared among other elements of the Fountayn Platform, an end-to-end clinical data management solution. Data can be exported for statistical analysis and electronic submission, in which a variety of formats for reporting are supported: Text, CSV, Excel, SAS and eSubmission PDFs.
Migrating from Paper to EDC - a Case Study
A major biotechnology sponsor was looking to accelerate timelines and cut costs in its clinical trials. Management committed to phasing in EDC studies and the process change needed to make the migration successful. At the time of this decision, the company was embarking on two 48-week paper- based pivotal clinical trials for an investigational compound, and because the study team had no experience with EDC, those studies were conducted with paper. Each study was originally intended to last two years and when protocol amendments were submitted to extend the studies out at least 8 years the decision was made to convert these paper studies to EDC using Fountayn. “These were very large international studies, so collecting and managing the data in paper would have been extremely difficult,” says Jill Fidgeon, former Project Manager for the migration to EDC.
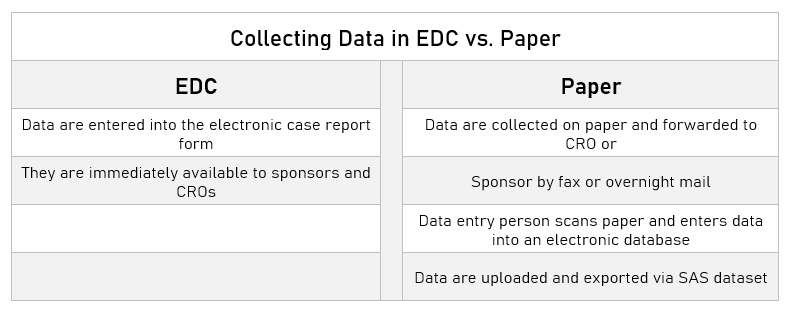
To launch the migration from paper to EDC, which started in July 2008 on the first paper study, the company prepared a grid of SAS datasets. The programmers began the task of taking each variable within those datasets and mapping it into the structure of the eClinical application. To confirm data integrity, a two-step process was implemented: first, the exported datasets were imported into the EDC application. Next, the data were exported from the EDC application as SAS datasets. The fact that the two resulting databases were identical was a measure of success. The entire process for the first study took several months and before the same process was applied to the second study an independent audit was conducted to ensure the process and resulting migration were sound – they were. The migration of the second study, using the same methodology took approximately 6 weeks and was completed in February 2009. A comparison of data collection in EDC vs. paper appears in Table 2. According to Bill Gluck, PhD, former Director Clinical Data Management, the benefits of EDC were immediately apparent. “From very early on, we found our ability to track and manage the safety profile of our drug was far superior using EDC as compared to the paper world.” Gluck explains that the paper-based pivotal trials had a monthly download of site data from the CRO, and monitors would visit the sites every 6-8 weeks and then every 8-12 weeks. As the studies progressed, the sponsor did not have current information, but with the switch to EDC, Gluck comments, “After patients came in for their visits, those data were entered into the system, and we did a data download every day.” With the daily refresh, the sponsor could see adverse events and their types and severity right away. If serious adverse events were spotted, but the site had not submitted the paperwork to comply with mandatory regulatory timelines, the company could proactively request the information. “With paper, at best, we would have been a month behind,” Gluck explains. Almost immediately, queries of omission were eliminated, as EDC prompts sites if data fields are left empty. In addition, once the EDC environment was implemented, the company could assign more studies per manager than previously as the burden of data management per study was reduced. Perhaps most significant was the fact that with both EDC studies, the database was locked in four weeks as compared to eight weeks in the paper studies. Fidgeon remarks, “Once everyone got used to EDC, many said that they never wanted to go back to paper. It saves so much time, and you can make informed decisions much earlier.”
1 Kreger J, Nadler N, Fatta R. Pharmaceutical Outsourcing, William Blair & Company, February 5, 2010
*Fountayn Formerly known as Datatrak