CTMS
Natively integrated with Datatrak EDC, RTSM, and eTMF.
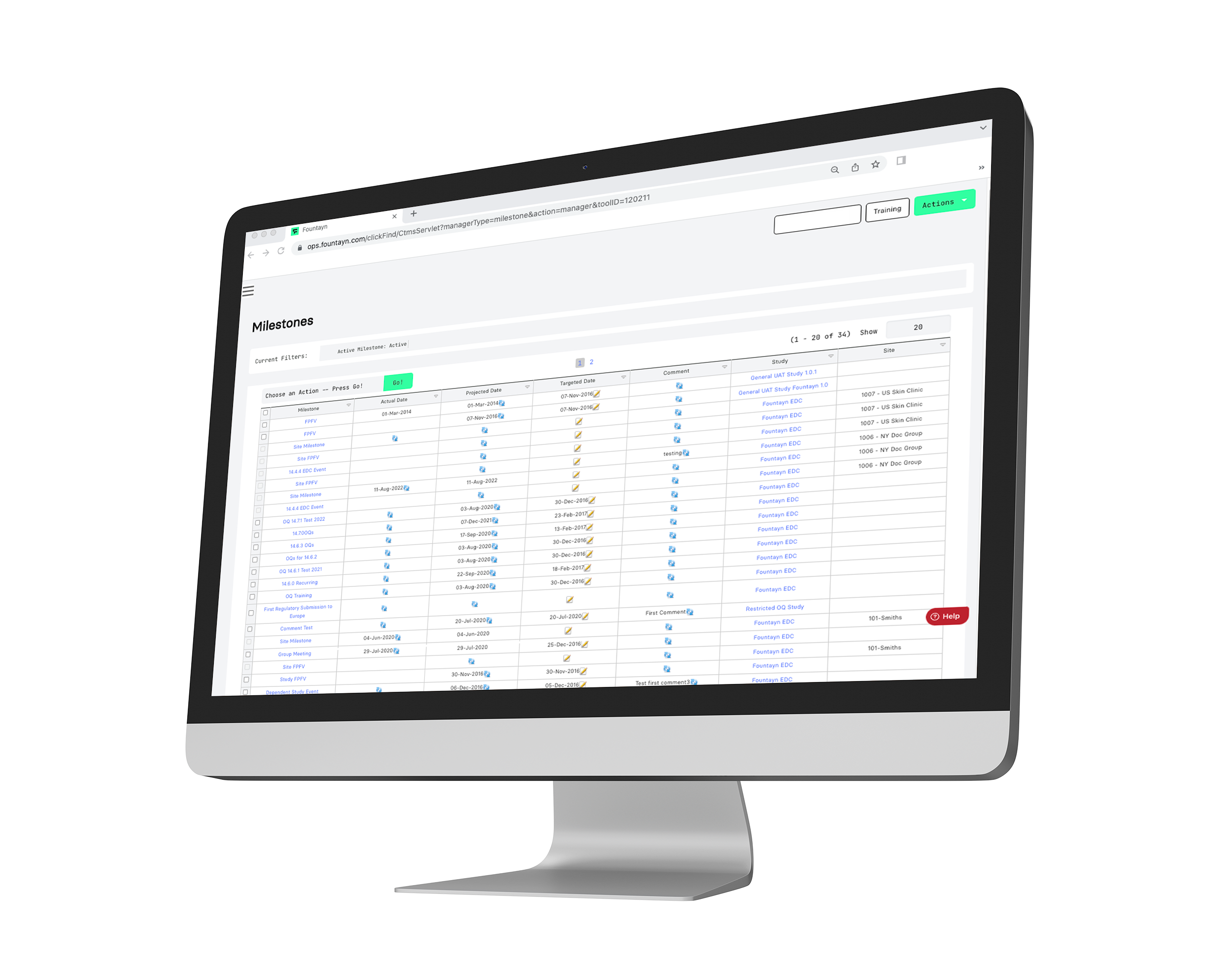
Fountayn's CTMS software optimizes your workflow with real-time insight-driven data and analytics, empowering users to accelerate the progress of clinical trials. View and control all clinical operation information and workflows for your study in one seamless and configurable platform.
Acting as a repository for clinical operations, our CTMS includes functions for site feasibility and the tracking of action items and milestones. Throughout our platform, we deliver your clinical trial data across our products proactively in real-time, ensuring your team has the most up-to-date information to accelerate progress.
Optimize Workflow
With intuitive dashboards and robust reporting capabilities, you can verify data across all studies in a single location.
Increase Monitor Productivity
Streamline the creation of monitoring reports by configuring a collection of templates that are easily duplicated and tailored by you.
Simplify Regulatory Submissions
Track and store relevant documents in a controlled environment and easily create checklists to manage your regulatory submission requirements.
Design & Configurability for Today’s World
Our intuitive, interface-driven configuration does not require technical skills or lengthy implementation on the user’s side.