Distinguishing our CTMS
Fountayn’s revolutionary CTMS (Clinical Trial Management System) redefines industry standards by leveraging the power of our Data Platform to enhance client functionality and client systems interoperability while significantly reducing their long-term cost of ownership.
CTMS Industry Background
The industries’ top four CTMS offerings grew historically through acquisition of stand-alone legacy systems that provided the basic functions of planning, performing, managing, and reporting on clinical trials. With the evolution of the Cloud and SaaS offerings, CRO’s and Pharma are now anticipating a more modern approach to CTMS.
Outdated legacy systems are easy to identify with requirements for internal hosting, upfront licensing fees, server procurement, vendor configuration, and ongoing customization for forms, workflows, reporting and more. Newer hybrid legacy systems are slightly better with web-enabled interfaces to servers hosted within the vendor’s remote hosting environment. Unfortunately, these offer the same pain points of limited adaptability, no real-time access to EDC systems (or the vendor’s EDC system), limited cross-study management functions, inefficient submission capabilities and APIs integrate only with the vendors own products with no or limited integration capability for client’s internal mission critical systems. Both offerings require long and expensive configuration, training, extensive validation, onboarding and implementation only to fail when restricted visibility is needed for study management to real-time data and performance measures.
The Emergence of CTMS by Fountayn
Fountayn’s CTMS solution originated from one central platform using the same Java coding and database that have successfully delivered thousands of clinical trials across multiple languages through our robust EDC solution. Fountayn CTMS is offered through our Enterprise Cloud in a SaaS business model without licensing fees, hosting requirements, long and expensive customizations, training and onboarding. We use our tested Java source code libraries, database platform, server environment, policies and procedures, testing automation, and enterprise platform to ensure our clients always have the latest and greatest versions.
CTMS Industry Background
Our goal for CTMS is to continue helping our clients get products to market faster from CTMS regulatory submissions to early stage EDC. To meet this goal, Fountayn CTMS was designed to give the clinical operations executives the business intelligence insights necessary to measure performance metrics and return on investment across development programs with real-time data. Ourflexible,configurableuser roles and permissions,combinedwithunlimiteduseraccounts, allowbothCROsandPharmatogiveaccessandvisibilitytoasmanyteam members as needed without affecting currentandfutureworkflows. Early evaluations by leading CROs, pharma and device companies indicate Fountayn CTMS is the best of breed solution. The following gap analysis provides a guide to critical features across the top five vendor offerings.
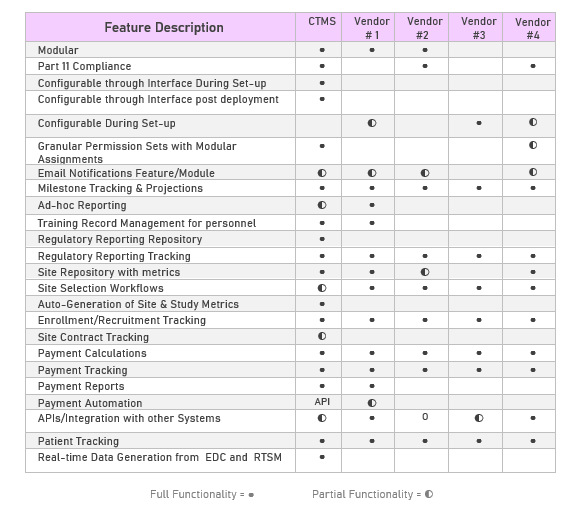
Standing up to Competing CTMS Systems
Differentiating Features in Fountayn CTMS
The scalable capabilities discussed here provide a higher level of workgroup collaboration for each organization’s needs (CRO’s, Regulatory, etc.) as they grow their enterprise. A key functional requirement was that Fountayn CTMS is designed to accept clinical trial data from any and all EDC systems without limiting CTMS functionality (a common problem with other systems). When used in conjunction with Fountayn EDC (which takes one click), our system will automatically populate all clinical trial data in real-time which includes all managers, dashboard performance metrics and reports.
CTMS is the only fully unified EDC to CTMS platform on the market.
We use one central database source for all EDC and CTMS. There is no transfer of data when using both the Fountayn EDC and CTMS. There is one common interface, with similar look and feel, and functionality across the user interface (including all language applications).
Forges real-time data, pulled directly from active EDC studies
Because of our SINGLE DATA SOURCE, CTMS is able to pull real-time data directly from our Fountayn EDC. Other companies rely on a sync, and movement of data, that needs to be regularly performed.
*Fountayn CTMS can also perform this sync with outside EDC vendors.
Pre-configured, turn-key solution that allows for same day use.
CTMS tools can be turned on within hours of contract signing. From there, configuring CTMS can be easily accomplished by non-technical users based on client’s needs.
HIGHLY Configurable Interface can be Adapted During Set-Up and Post-Deployment
No technical experience needed. Virtually all data collection forms can be freely reconfigured to show the values you require. For example you could easily add another drop down menu or remove “fax” if you do not want it. If you change your mind later, you will be able to update the configuration post initial deployment without downtime or technical support.
Includes a first-of-its-kind Regulatory Submission Manager
Fountayn harnesses the power of technology to simplify the process of regulatory submissions. Our modern technology provides actionable data to improve workflows, reduce human error, and provide greater trial team efficiency. This process allows you to eliminate redundancy, providing an opportunity to reuse common elements in submissions across all studies
Payment Reports to accommodate site payments
Track site payments issued for site specific budget items and patient activity. These patient visit payments can be triggered automatically based on patient data. Visits recorded within our EDC and automatically populated within CTMS. Pull patients information from other EDC via import or our easy to use APIs.
FLEXIBLE permission sets with dynamic or granular permissions that grow with your database.
With CTMS by Fountayn, there are two types of users:
Restricted users only can see what is set up for them to see. Dynamic permissions entities are labeled subset of data in the system. These can be defined as sponsor, CRO and Country. For example, a CRO would only see the sites they are assigned to. Permissions automatically grow as the data in CTMS is expanded. Granular permissions can be used if more specific permissions are needed, and give you the ability to give permissions to a role that are as specific as a single study.
The second type of user is the Unrestricted Users, who can see everything on the screens that they are given access to.
Utilize APIs to connect to any system used in your Clinical trial
Multiple formats are available to get your data into Fountayn’s CTMS with secure and automated Application Programming Interface (API).
Default Monitoring Reports
Templates can be copied to become new template records, then saved to add new record to the manager. Connected fields can be automatically populated with data from CTM, or the EDC study to which it is linked. All reports are version controlled, and can be modified at any time.
Conclusion
CTMS by Fountayn transcends organizational boundaries, improves interoperability, and scales with evolving regulatory standards. It maintains and manages clinical trial planning, preparation, performance and reporting, with an emphasis on keeping up-to-date contact information for trial participants and tracking deadlines and milestones. The scale of the clinical trials industry means that even small gains in efficiency can have profound implications for the organization and ultimately the patients they serve. Leveraging the unifying trend of technology in the digital clinical space, we can drive down costs and improve efficiencies by eliminating redundancy, reducing user errors, improving workflow, and delivering actionable data that provides meaningful insight.
*Fountayn Formerly Known as Datatrak